 |
UPI Senior Medical Correspondent Washington (UPI) May 09, 2007 Despite the limitations on federal funding for embryonic stem cell research, two companies recently said they are close to entering clinical trials with the versatile cells. Geron plans to file an investigational new drug application with the Food and Drug Administration by the end of the year for using cells derived from embryonic stem cells for treating spinal injuries. Advanced Cell Technology, which previously said it planned to file an IND this year for using stem cell-derived therapies for treating macular degeneration, announced this week it has developed a technique to generate a type of progenitor cell that could move into the clinic in 2008 for treating a variety of ills. Robert Lanza, Advanced Cell's vice president of medical and scientific affairs, told United Press International that the cells -- called hemangioblasts that his group derived from human embryonic stem cells -- have proven their ability to repair vascular damage in the eyes and limbs of animals. This indicates the cells could prove beneficial for treating heart attacks, reversing vascular damage that now requires limbs to be amputated, and other conditions. "We're planning to file with the FDA next year to use them in patients," Lanza said. Advanced Cell's technique is described in the online issue of Nature Methods. Although it's still in the early days, he said the hemangioblasts also could be used to create immune tolerance so the body does not reject the cells as foreign. "This would allow us to transplant any type of replacement cell or organ generated from a specific stem cell line without rejection," Lanza said. "It would make therapeutic cloning unnecessary and obviate the need for millions of human eggs." Lanza said animal studies his firm currently has in progress indicate the hemangioblasts could help repair lung damage and generate enough red blood cells for transfusion. Other potential indications include treating strokes, microvascular complications of diabetes and atherosclerosis. Advanced Cell, whose California facility could be a benefactor of the $3 billion stem cell program in that state, also may reap the rewards on the other coast where its Worcester, Mass.-based facility is located. Massachusetts Gov. Deval Patrick Tuesday announced his proposal to make $1.25 billion available for funding stem cell and other research in the state over 10 years. Under the terms of the proposal, the majority of the funding would come from the state, while $250 million would come from private businesses. UPI could not reach Geron CEO Thomas Okarma by press time Wednesday, but the company has said it anticipate filing an IND for GRNOPC1 for treating spinal-cord injuries around the December timeframe. GRNOPC1, which consists of oligodendroglial progenitor cells derived from human embryonic stem cells, has been shown to stimulate the regeneration of damaged neurons in pre-clinical studies. Lazard analyst Joel Sendek, who rates the stock a "hold," notes Geron's products, since they are cellular-based therapies, carry substantially more risk than conventional drugs or protein therapies. Despite that uncertainty, the company's GRNOPC1 may have an advantage over stem cell-based therapies aimed at other indications. "We believe the bar for signs of efficacy is low, given that (spinal-cord injury) patients have no other options for restoration of function," Sendek stated in a research report. However, the FDA is concerned about the potential for stem cell-derived therapies to cause tumors in humans, so Geron will have to overcome that barrier with the agency, Sendek said. He anticipates the company will file the IND for GRNOPC1 in the fourth quarter and start a phase 1/2 program in the first half of 2008. The phase 1/2a trial, which Sendek anticipates will take two years to complete, will initially involve 75 patients with spinal-cord injuries. GRNOPC1 cells will be injected into the spinal-cord lesion and the patients will also be given an immunosuppressant drug to prevent rejection of the cells. Mark Monane, an analyst with Needham, thinks the IND filing for GRNOPC1 and advancement of its other pipeline candidates will be significant events for Geron, but added they probably won't add much value to the stock. "Given the current technology value of $288 million, we believe that the market has already priced in the expected pipeline progression," Monane stated in a research report. "Going forward, we believe that the stock will perform in line with the overall market until (generation of) further clinical efficacy data from Geron's multiple product candidates." The company's other candidates include GRN163L for chronic lymphocytic leukemia. A potential catalyst for the stock is Geron's slated presentation of early phase 1/2 data for GRN163L at the Pan Pacific Lymphoma Conference in June.
Source: United Press International Related Links Advanced Cell Technology The Clone Age - Cloning, Stem Cells, Space Medicine
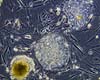 Washington (UPI) April 11, 2007
Washington (UPI) April 11, 2007The Senate moved Wednesday to repeal the Bush administration's restrictions on embryonic stem cell research, marking the second time in less than a year Congress has voted by wide bipartisan margins to reverse the president. |
|
| The content herein, unless otherwise known to be public domain, are Copyright Space.TV Corporation. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement, agreement or approval of any opinions, statements or information provided by Space.TV Corp on any Web page published or hosted by Space.TV Corp. Privacy Statement |