 |
UPI Correspondent Washington (UPI) April 09, 2007 While the future for personalized medicine has far exceeded expectations, funding is critical in order to continue taking the necessary steps, health experts said Monday. "We are on a real roll here in terms of the ability to discover things that we previously thought would take us a decade or more, which are in fact turning out at remarkable speed," said Francis Collins, director of the National Human Genome Research Institute. Collins emphasized the April 2003 completion of the Human Genome Project, which incorporated more than 2,000 scientists from six different countries to achieve the common goal of mapping out all the letters of the "human DNA instruction book." That research created a platform to launch new applications of the technology that predict medical problems in people via personalized medicine. Personalized medicine uses a person's genetic makeup -- specifically their genome or DNA -- to create individualized methods of treatment for diseases. By studying the 3 billion letters within the human genome and the genetic variations within it, scientists can detect, treat and even prevent disease. In 2002, the HapMap project created a map that specified how human genomes are organized, which enabled scientists to identify specific genes that affect a person's susceptibility to certain diseases including diabetes, cancer, heart disease, and blindness. The HapMap process has already led scientists to discover certain genes that determine whether or not someone will negatively respond to medication they're given. One example is the blood thinner warafin, which can now be adjusted in specific doses, based on a person's susceptibility to the drug. Several other drugs have been marketed to treat cancer patients through the same process. HapMap will soon play a role in identifying risks of diabetes in young people, health experts said. This increasing technology is leading the National Institutes of Health to make a bigger investment of tax dollars for genomic medicine and research, said Elizabeth Nabel, director of the National Heart, Lung, and Blood Institute, part of the NIH. "We have invested considerable monies in our institute portfolio into trying to realize the promise of genomic, personalized medicine," Nabel said. Currently, the NIH is investing in a heart disease study, Framingham SHARe, in order to discover genes causing cardiovascular diseases and disorders such as osteoporosis and diabetes. Approximately 10,000 people are taking part in repeated exams, with measures called phenotypes -- such as height, weight, cholesterol level -- recorded and put into a database. The study volunteers have also agreed to have their DNA studied, which will be the key aspect of the study: identifying genetic sequences and recognizing common variants. The understanding of people's phenotypes will help "form associations between genetic background and clinical manifestations of health or disease," Nabel said. While this process expands the potential to treat future diseases, it's also raised questions about ethics of genomic medicine, especially privacy issues. Although the existence of a medical database containing people's DNA is essential for scientists to gain access to and study the genomes, the information could possibly fall into the hands of health insurers or employers. Sharon Terry, president of Coalition for Genetic Fairness, has spent 12 years working on the Genetic Information Nondiscrimination Act, which would prevent genetic discrimination through legislative protection in order to ensure that people's employers can't fire them or insurance agencies refuse to cover them due to a risky genetic profile. But some employers and insurance companies, as part of the Chamber of Commerce and the National Association of Manufacturers, are speaking out against the bill, saying businesses shouldn't be forced to employ or give coverage to people with a genetic disease. The bill has passed unanimously in the Senate, but has so far failed to muster enough votes in the House. "Our fear is that members of Congress don't understand the importance of the bill," Terry said. But while the two sides continue to debate the ethical issues, the cost hurdles that once stymied personalized medicine are starting to crumble. Specifically, in 2002, it would have cost about $10 billion to examine someone's entire genome for just one human disease. But just five years later, it costs less than $1 million. This method will continue to be applied to many more common illnesses as the price continues to fall, experts predict. While the timetable of genomic medicine isn't certain, NHGRI's Collins said he is optimistic that, in five to seven years, patients will "have this kind of broader availability of predicted genetic testing."
Source: United Press International Related Links Hospital and Medical News at InternDaily.com Hospital and Medical News at InternDaily.com
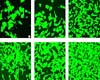 Providence RI (SPX) Apr 10, 2007
Providence RI (SPX) Apr 10, 2007Biomedical engineers are constantly coming up with ways to repair the human body, replacing defective and worn out parts with plastic, titanium, and ceramic substitutes - but the body does not always accept such substitutes seamlessly. |
|
| The content herein, unless otherwise known to be public domain, are Copyright Space.TV Corporation. AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement, agreement or approval of any opinions, statements or information provided by Space.TV Corp on any Web page published or hosted by Space.TV Corp. Privacy Statement |